CHIP is advancing the incorporation of genomic and other omics data into the practice of medicine through discovery and new care models. Medicine investigates the complex DNA of an individual for effective diagnosis and tailored treatment.
Our program has worked with sister academic medical institutions to share genomic data on broadly consented cohorts, across sites of care. We have, in partnership with our sister sites, developed two federated genomic data sharing commons, the Genomic Information Commons (GIC) and the Genomic Research and Innovation Network (GRIN).
Projects

Inspired by a common vision of accelerated genomic discovery, collaboration, and improved clinical outcomes, leaders at Boston Children's Hospital, Cincinnati Children's Hospital Medical Center, the Children's Hospital of Philadelphia, Washington University at St. Louis, and the University of Pittsburgh Medical Center have come together to create the Genomic Information Commons (GIC). The GIC is an NCATS-funded, multi-institutional, federated, genomic data commons.

Three of the nation’s leading children’s hospitals launched the Genomic Research and Innovation Network (GRIN), with federated information technology infrastructure, harmonized biobanking protocols, and material transfer agreements. Harmonized, broadly consented institutional review board (IRB) protocols were approved and used for biobank enrollment, creating ever- expanding, compatible biobanks. An open source federated query infrastructure was established over genotype–phenotype databases at the three hospitals. Investigators securely access the GRIN platform for prep to research queries, receiving aggregate counts of patients with particular phenotypes or genotypes in each biobank.

CHIP directs the Biobank Core at Boston Children’s Hospital which includes the Precision Link Health Discovery protocol for engaging our patients in research and capturing the diversity of our patient population under a broad consent for omics and phenotype data phenotype biospecimens stored in the biobank core.
The Clinical Pharmacogenomics Service (PGx Clinic) works to make medications safer and more effective by incorporating patient’s genetic information when making decisions about medication choices. The PGx Clinic applies genetics to determine variations in medication response to improve clinical care.

Autism is caused by gene x environment interaction during brain development. We aim to investigate what contributed a significant increase to the autism prevalence in the U.S. over the past decades, focusing on discovery of internal and external environmental exposures that are detectable in the blood from the patients with autism and their family members compared to individuals without autism.
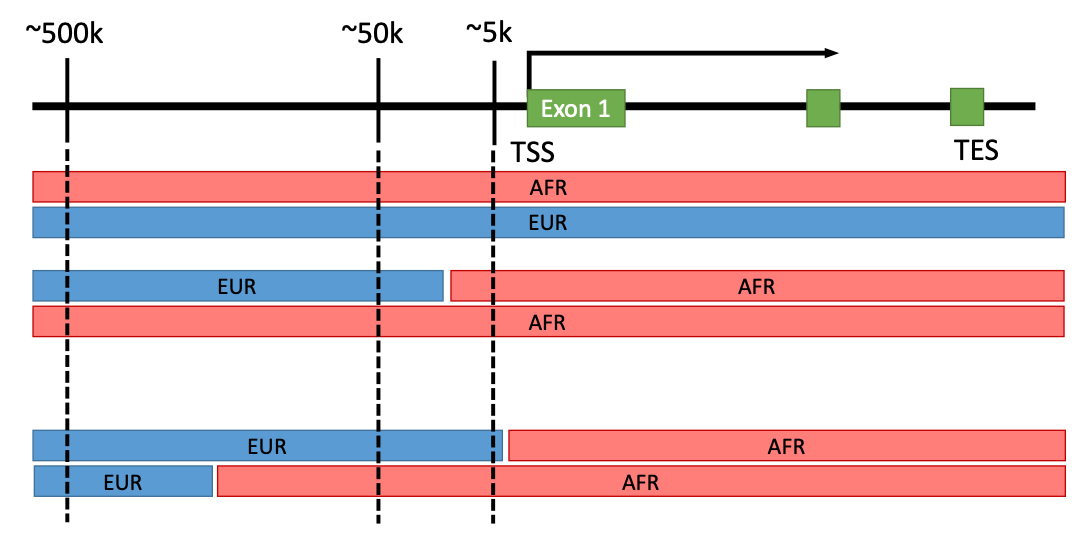
Regulation of gene expression is complex and non-coding regions of human genome have diverse regulatory elements. We hypothesize that local ancestry specific haplotypes containing regulatory elements have impacts on (local-) ancestry specific difference in gene expression regulation. 70% of human genome may include regulatory elements of which some are unique to ancestry-specific haplotypes.
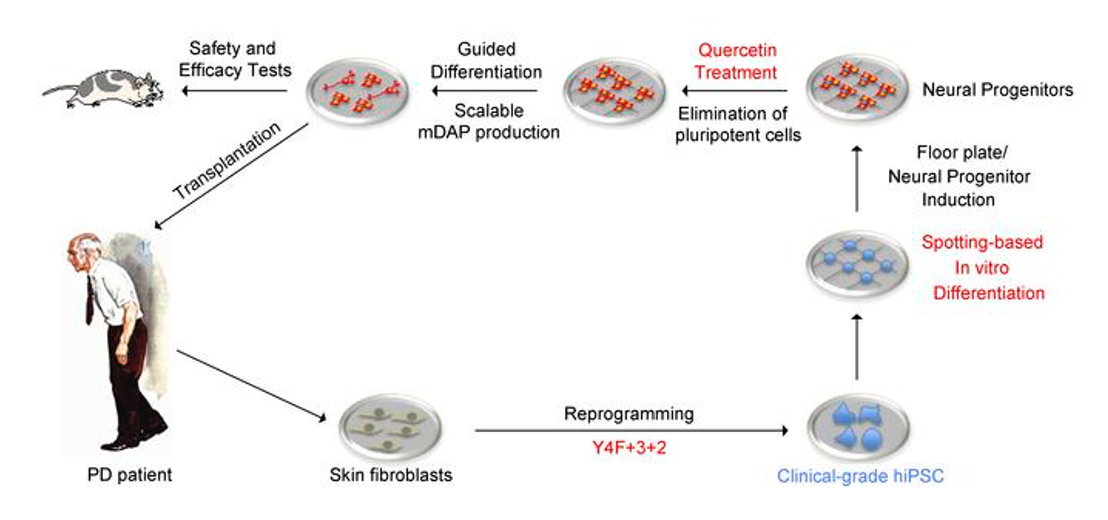
Parkinson’s disease (PD) is the second most prevalent neurodegenerative disorder and caused by the loss of functional dopaminergic neurons in substantia nigra. Therefore, a cure is to transplant dopaminergic neurons that are ideally generated using autologous cells. We successfully treated a patient with PD. An outstanding challenge is to generate clinical-grade iPSC lines without potentially harmful somatic mutations. We are establishing and optimizing protocols to ensure genome integrity and free of mutations for clinical trials.
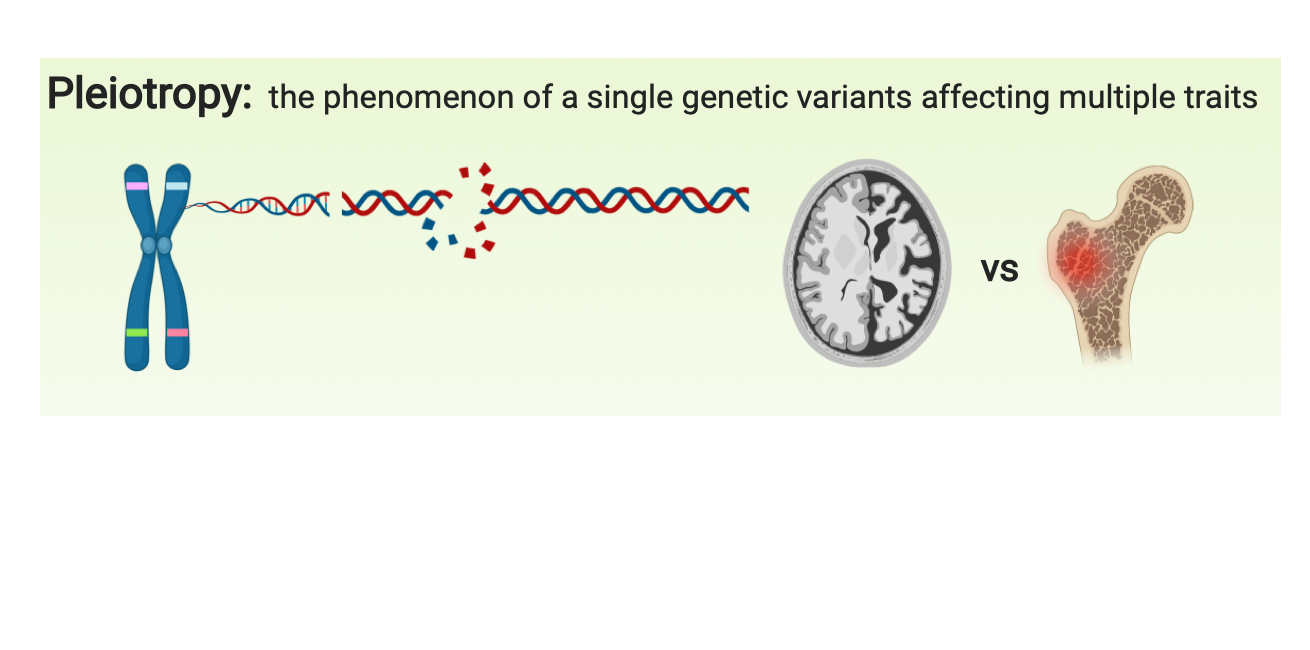
Tissue-resident macrophages are highly specialized to their tissue-specific microenvironments and activated by various inflammatory signals and modulated by genetic and environmental factors. Osteoclasts and microglia are distinct tissue-resident macrophages for bone and brain that are responsible for pathological changes in osteoporosis and Alzheimer’s disease (AD), respectively. Pleiotropy is the phenomenon that a genetic variant affects multiple traits. We discover the genes with pleiotropy affecting brain and bone by focusing on tissue-resident macrophages.